I – STUDY OF THE DIFFERENCES OF FORMATIVE FORCES DURING DAY OR NIGHT
with the help of Silver nitrate (1%) Iron sulphate (1%) Equal quantities mixed of: 1% Silver nitrate and 1% Iron sulphate. Equal quantities mixed of: 1% Silver nitrate, 1% Iron sulphate and 1% Lead nitrate.
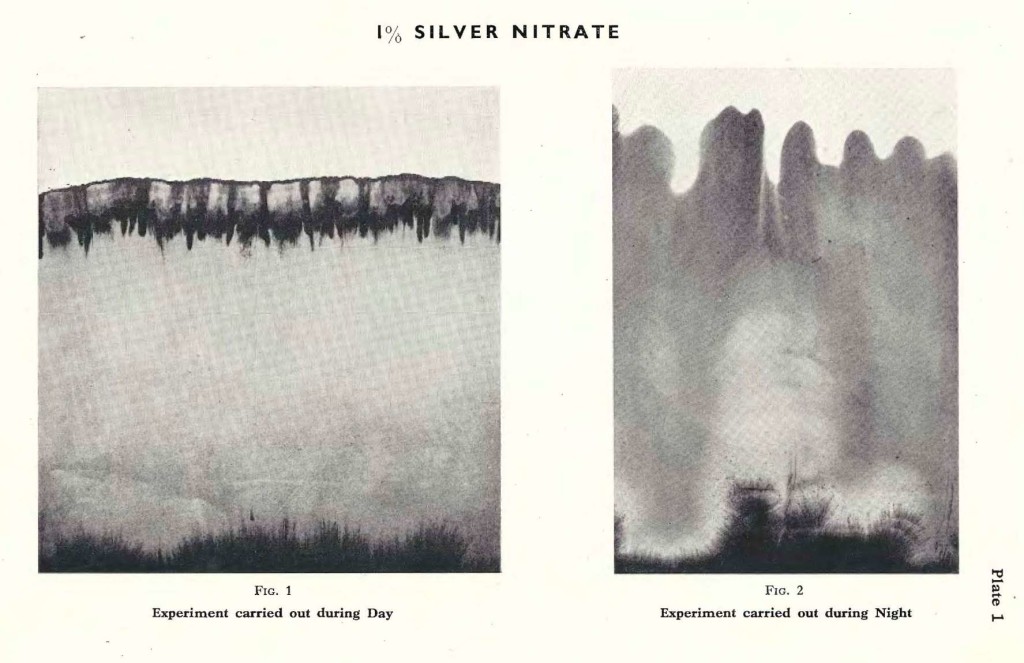
As described previously the filter paper is immersed in a 1% solution of Silver nitrate; the latter is allowed to rise until it comes to a stop of its own accord. This does not necessarily mean that the whole amount of 10 cc. has been absorbed. It does not matter that the filter paper remains immersed in the solution. The rising height will remain unaltered, unless there occurs a sudden change in the temperature or humidity of the atmosphere. The experiment is carried out during day time, fully exposed to the influence of the sun. After the rising activity has come to an end, the filter paper begins to dry from the border line downwards, and slowly turns light brown,and, later, darker brown. The border forms a specific pattern, similar to the one depicted on Plate 1, Fig. 1.
 To facilitate the study of these experiments all the pictures are printed on single sheets, which can easily be taken out of the folder at the back of the book and spread out on a table.
To facilitate the study of these experiments all the pictures are printed on single sheets, which can easily be taken out of the folder at the back of the book and spread out on a table.
Fig. 1 is a typical example of Silver nitrate, forming its characteristic pattern during day time. Of course variations occur in the rising height, especially on a rainy day;or in the colour, if the experiment is carried out in midsummer when the sun is shining down fiercely, speeding up the process of reduction.
The same experiment may be carried out during night. It is done in the same room, at the same spot. The only difference is, that instead of the Sun, eventually the Moon may shine on the filter paper into which the solution is rising. Or, in a dark night, not even the Moon gives light. As a rule, the liquid rises higher, the temperature being lower and the humidity increased, compared with conditions during day time.Since there is no light, or only very little, the silver is scarcely reduced at all. In the morning we find therefore a very pale brown pattern, which darkens with the increasing day light.
The pattern formed during the night is definitely different from the one formed during the day. Plate 1, Fig. 2 is a typical example of an experiment with Silver nitrate carried out during the night. Comparing Fig. 1 with Fig. 2 it is obvious that the Silver nitrate has risen considerably higher, that the border line is less sharply formed. Soft waves run across horizontally, whereas the day experiment forms a broad band intersected by vertically pointed darker lines or fringes.
Experiments carried out during the day or during the night are fundamentally different, and we may try to explain this by the presence or absence of light, the difference in temperature and humidity. Light especially seems an important factor where silver salts are concerned. In our publication of 1929 we gave examples of experiments carried out during the day in a dark room, but we regret that it is impossible to go into all these details here.
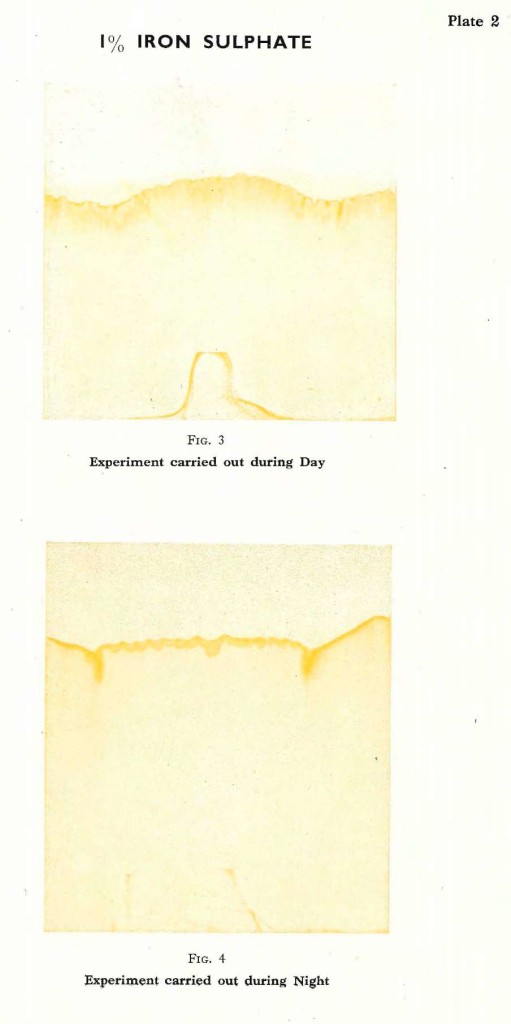
Let us turn our attention to another salt, where the influence of light does not play such an important part, and study the differences between an experiment carried out during the day or during the night. For instance it is possible to take Iron sulphate.The crystals of Iron sulphate are light green and dissolve readily in water. Again a1% solution is used. The experiment carried out in bright daylight shows the liquid rising, coming to a natural limit, and the filter paper slowly turns ochre yellow. The border line is well formed, intersected by numerous vertically pointed lines, but much more subtly formed than those observed in the experiment with Silver nitrate. In time this pattern will come out even more sharply, the yellow will darken to a deeper ochre tone. No further changes occur and such an experiment can be kept for an indefinite period. The experiment with Silver nitrate can only be kept a short while, even in a cool and dark room; the silver gets darker and darker and the characteristic forms will eventually vanish completely.
The experiment with Iron sulphate is repeated during the night and again it can be observed, that the solution rises higher than during the day time. The pattern formed is much softer, compared with the day experiment, although different from the waves formed by Silver nitrate. The colours are just the same as those produced during the day and have the same tendency to darken slightly in time. But they never darken beyond a rich ochre yellow. Fig. 3 and Fig. 4 on Plate 2 are typical examples for such experiments with Iron sulphate.
 A further step leads us to attempt a mixture of Iron sulphate and silver nitrate,using again 1%- solutions and equal quantities. A chemical reaction takes place between those two substances and a greyish deposit is formed. The liquid rises and after a few minutes a tiny black spot appears on the filter paper, and then another and another sometimes with great speed, sometimes slowly, these tiny black spots grow and form peculiarly shaped arrows. The strange thing is, that, looking at the finished experiment,one is inclined to think that these arrows have been formed from the top downwards,but the opposite thing has happened in reality. The dark point of the arrow appeared at first and slowly opened up, growing from the bottom towards the top. This peculiar arrow-like structure is characteristic for a mixture of Silver nitrate and Iron sulphate.Neither the silver nor the iron alone can produce it, only the two together. The dark,nearly black colour is of course due to the Silver nitrate but the form is born out of the interaction of the two metal salts. Plate 3, Fig. 5 is a typical example of an experiment carried out during daytime. The presence of Iron sulphate in the mixture has in away speeded up the reduction of the silver salt. The light parts show the yellow colour due to the Iron sulphate.
A further step leads us to attempt a mixture of Iron sulphate and silver nitrate,using again 1%- solutions and equal quantities. A chemical reaction takes place between those two substances and a greyish deposit is formed. The liquid rises and after a few minutes a tiny black spot appears on the filter paper, and then another and another sometimes with great speed, sometimes slowly, these tiny black spots grow and form peculiarly shaped arrows. The strange thing is, that, looking at the finished experiment,one is inclined to think that these arrows have been formed from the top downwards,but the opposite thing has happened in reality. The dark point of the arrow appeared at first and slowly opened up, growing from the bottom towards the top. This peculiar arrow-like structure is characteristic for a mixture of Silver nitrate and Iron sulphate.Neither the silver nor the iron alone can produce it, only the two together. The dark,nearly black colour is of course due to the Silver nitrate but the form is born out of the interaction of the two metal salts. Plate 3, Fig. 5 is a typical example of an experiment carried out during daytime. The presence of Iron sulphate in the mixture has in away speeded up the reduction of the silver salt. The light parts show the yellow colour due to the Iron sulphate.